
Swiss LithoClast Trilogy: A European Multicenter Prospective Study
Study overview
- Ten European clinical centers participate in this prospective, non-randomized trial.
- Each site has the goal to provide data on 20 cases (total: 200)
- Principal investigator is Oliver Wiseman, Cambridge UK.
- The goal of the study was to provide objective data for stone clearance rate, stone-free rate, complications and device malfunction; as well as subjective usability data such as ergonomics
First publication available
Interim results of 53 patients were accepted for presentation at EAU Amsterdam and AUA Washington, but unfortunately, these congresses were cancelled. Fortunately, the first publication has been made available now:
O Wiseman et al,: The Efficacy And Safety Of The Ems Lithoclast Trilogy: A European Multicenter Prospective Study On Behalf Of ESUT
Weblink: https://www.auajournals.org/doi/abs/10.1097/JU.0000000000000855.011
First presentation at 40th Congress of the Société Internationale d`Urologie
- Inclusion of 157 case studies confirming high post-OP stone free rate (Fluoroscopy: 83%, visual: 90%)
- Confirmation, that 24 out of the 157 cases were performed using a miniaturised technique with a median track size of 17.5 FR and median Trilogy probe size of 1.5mm
Key takeaways:
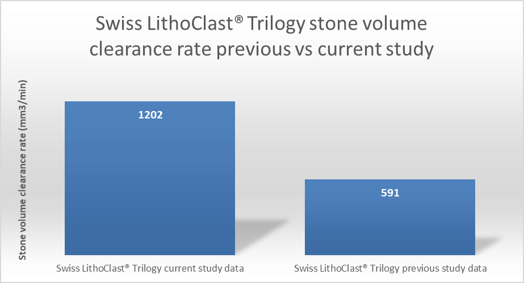
- The stone removal efficiency of Trilogy is almost 100% greater compared to published data on other Lithotrites.
- Post-OP stone free rate: 88%
- The device was perceived by surgeons to be highly effective overall and compared to the most commonly used previous lithotrite.
- In addition, the study confirms that Swiss LithoClast Trilogy is highly effective and safe for miniaturised PCNL
Multicenter study confirms ease of use:
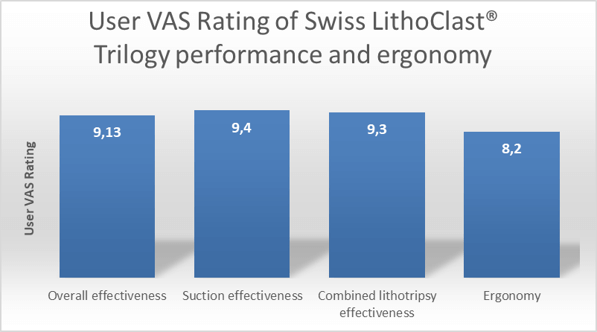
Multicenter study reports twice the effectiveness than previously published study data :
Related products
Swiss LithoClast® Trilogy
Experience lithotripsy like never before
Swiss LithoClast® Trilogy Handpiece
Ballistic, Ultrasound and Suction: 3 Modalities via one single probe


